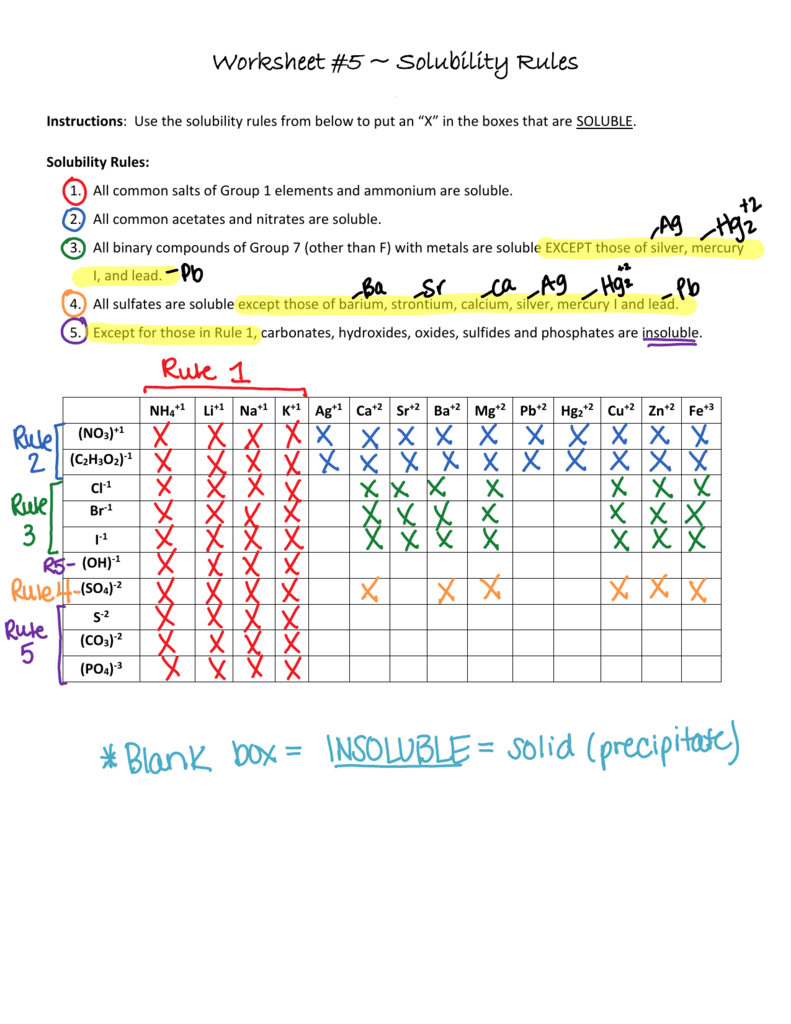
Solubility Rules Worksheet KEY
Solubility Rules and Common Ions Solubility Rules NO3-1 All nitrates are soluble. Cl-1 All chlorides are soluble except: AgCl, PbCl2, and Hg2Cl2. SO4-2 All sulfates are soluble except: Ag2SO4, PbSO4, Hg2SO4, CaSO4, SrSO4, and BaSO4. CO3-2 The carbonate of Group 1 metals and (NH4)2CO3 are soluble. All other carbonates are insoluble. OH-1 The hydroxides of Group 1 metals, and the heavier Group 2
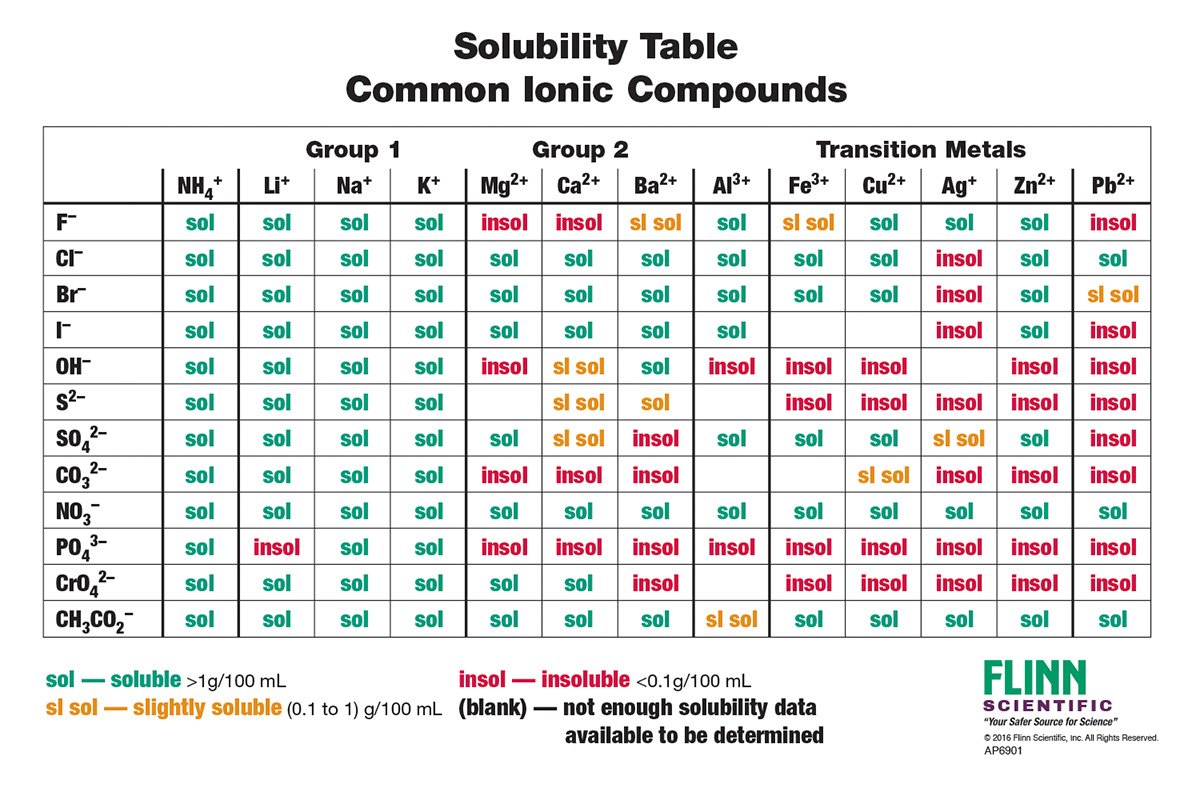
Solubility Rules Charts for Chemistry
12. Introduction to Organic Chemistry 1h 6m. 13. Alkenes, Alkynes, and Aromatic Compounds 1h 23m. 14. Compounds with Oxygen or Sulfur 31m. 15. Aldehydes and Ketones 37m. Learn Solubility Rules with free step-by-step video explanations and practice problems by experienced tutors.

Solubility Rules Practice Worksheet worksheet
Calculate the solubility in moles/L of each of three salts and the concentration of the cations in mg/mL in each of the saturated solutions. AgCN A g C N with Ksp = 2.0 ×10‐12 K s p = 2.0 × 10 ‐ 12. BaSO4 B a S O 4 with Ksp = 1.5 ×10‐9 K s p = 1.5 × 10 ‐ 9. FeS F e S with Ksp = 3.7 ×10‐19 K s p = 3.7 × 10 ‐ 19.

Solubility Rules Doodle Notes Doodle notes, Chemistry activities, Fun
Calculate the solubility in moles/L of each of three salts and the concentration of the cations in mg/mL in each of the saturated solutions. AgCN. A g C N. with Ksp = 2.0 × 10 ‐ 12. K s p = 2.0 × 10 ‐ 12. BaSO4.

(CHEM 1407) Solubility Rules Worksheet Answers
Solubility Rules Worksheet. Name or give the chemical formula for each of the following compounds. State whether they are soluble (will dissolve) or insoluble (will not dissolve) in solution. Use solubility rules. Chemical Formula. Name. Solubility. 1. NH4CH3COO.

19 Best Images of Importance Of Following Rules Worksheet Following
2AgNO 3 + Na 2 S → Ag 2 S + 2NaNO 3. A precipitate form if either Ag 2 S or NaNO 3 is insoluble. From the solubility rules, sulfides tend to be insoluble, so Ag 2 S likely forms a precipitate. NaNO 3 is soluble and does not form a precipitate because most nitrates are soluble. Since Ag 2 S forms a precipitate, one does form in this reaction.

17 Who Rules Worksheet Answers /
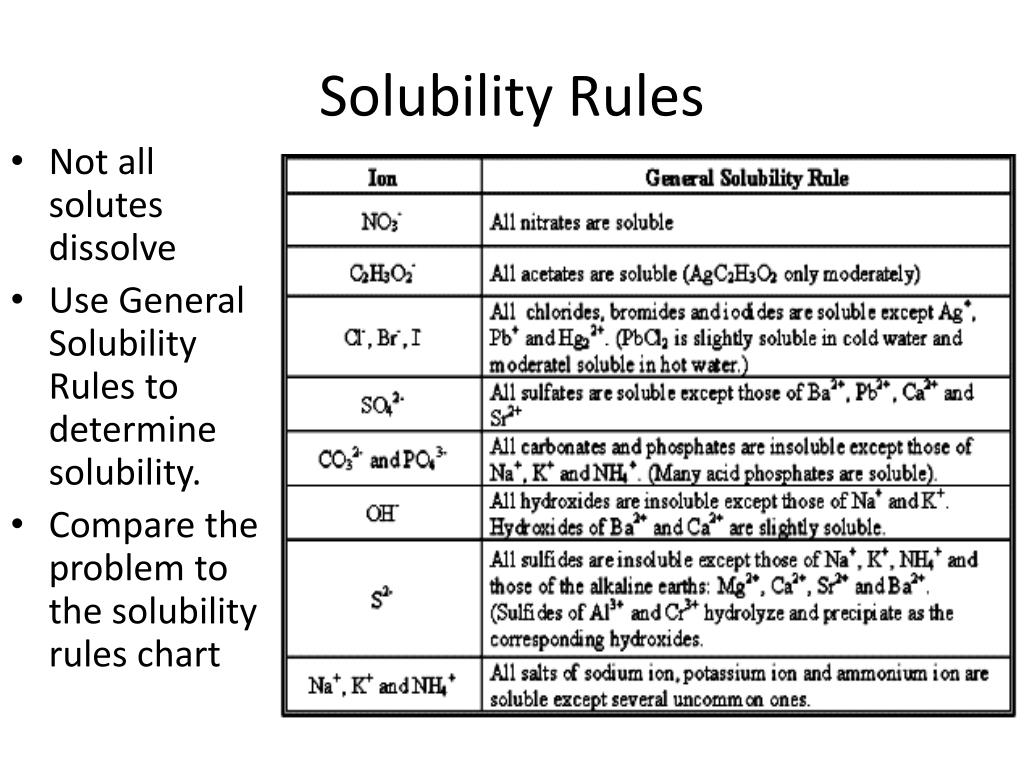
Salts containing Cl -, Br -, or I - are generally soluble. Important exceptions to this rule are halide salts of Ag+, Pb2+, and (Hg2)2+. Thus, AgCl, PbBr2, and Hg2Cl2 are insoluble. Most silver salts are insoluble. AgNO3 and Ag (C2H3O2) are common soluble salts of silver; virtually all others are insoluble.
15 Solubility Rules Worksheet Answers /
Generally Soluble. Carbonates. Phosphates. Oxides. Sulfides. Generally insoluble. Except when with ammonium (NH4) Potassium (K) and Sodium (Na) Hydroxides. Insoluble.

Unit VIB Solubility Rules Worksheet
Because Rule #3 precedes Rule #4, the compound is insoluble and will form a precipitate. 4. Predict whether a precipitate will form as a result of this reaction: 2AgNO3 + Na2S → Ag2S + 2NaNO3 (1) (1) 2 A g N O 3 + N a 2 S → A g 2 S + 2 N a N O 3. The products of the reaction must be examined; if either of the substances formed in the.

1. Solubility Rules Practice Worksheet State whether the following
Information (Solubility Rules) A solute is considered soluble if an appreciable amount of it can be dissolved in a given amount of the solvent. For example, both table salt (NaCl) and table sugar (C11H22O11) are soluble substances in water. A solute is considered insoluble if very little of it dissolves in a given amount of the solvent.

Solubility Worksheet Answers worksheet
Solubility Rules. A solute is considered soluble if an appreciable amount of it can be dissolved in a given amount of the solvent. For example, both table salt (NaCl) and table sugar (C 11 H 22 O 11) are soluble substances in water.A solute is considered insoluble if very little of it dissolves in a given amount of the solvent. For example, sand (SiO 2) is considered insoluble in water.

solubility rules practice worksheet Cindy Diagram
from 30 to 100. Slightly soluble. from 100 to 1,000. Very slightly soluble. from 1,000 to 10,000. Practically insoluble. more than 10,000. Review the solubility rules for common ionic compounds in water and download our solubility rules chart for easy reference. Look up compounds like calcium carbonate, barium sulfate, and sodium sulfate.

Use this worksheet for chemistry engagement! Students will enjoy
10. Periodic Properties of the Elements 1h 47m. The Electron Configuration 10m. The Electron Configuration: Condensed 2m. The Electron Configurations: Exceptions 9m. The Electron Configuration: Ions 12m. Paramagnetism and Diamagnetism 4m. The Electron Configuration: Quantum Numbers 7m. Valence Electrons of Elements 8m.

Solubility Rules Chart in Word and Pdf formats page 2 of 2
How soluble is it? Not all soluble substances dissolve equally. In this science worksheet, your child learns about differing rates of solubility by reading a bar chart and interpreting the data to answer questions. SCIENCE | GRADE: 5th. Print full size.

Solubility Rules Practice Worksheet worksheet
Use solubility rules. Chemical Formula Name Solubility 1. NH 4CH 3COO 2. Ba(OH) 2 3. Iron (II) Carbonate 4. NaOH 5. RbNO 3 6. Cesium Sulfate 7. MgSO 4 8. ZnCl 2 9. Zinc Hydroxide. solubility rules worksheet Author: pinar m alscher Created Date: 9/27/2017 4:10:52 AM.
PPT Solubility Rules PowerPoint Presentation, free download ID5256674
Solubility Rules Practice Worksheet 1. Name or give the chemical formula for each of the following compounds. 2. State whether they are soluble (will dissolve) or insoluble (will not dissolve) in solution. Use solubility rules. Chemical Formula Name Solubility 1. NH 4 C 2 H 3 O 2 2. Ba(OH) 2 3. Iron (II) Carbonate 4. NaOH